Research study participants are more engaged when they see their test results
body copy
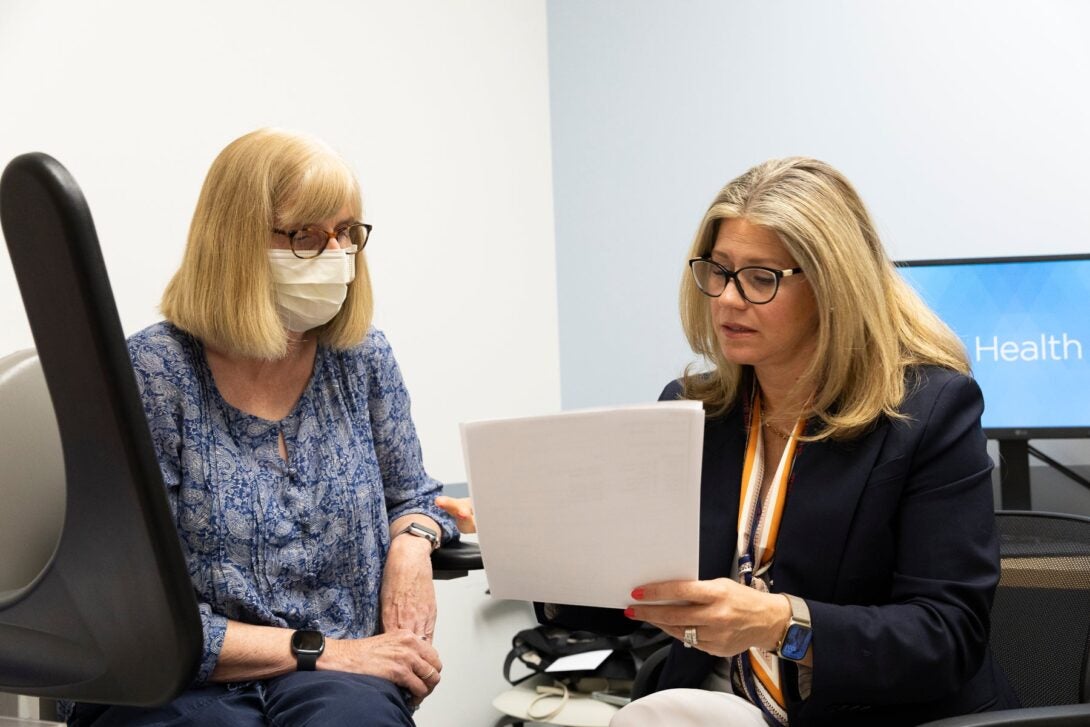
Enabling participants in clinical trials to see their medical test results can boost enrollment and engagement in research studies, according to a new paper from researchers at the University of Illinois Chicago. The research, conducted as part of a long-COVID study, found that returning results to participants can also create secondary health benefits.
Researchers from the UIC colleges of Nursing and Medicine interviewed volunteers in the Illinois branch of the RECOVER initiative — a national study of people diagnosed with long COVID — about their desire to receive the results of medical tests performed during the study.
The results, published in the Journal of Clinical and Translational Science, found that two-thirds of participants valued receiving the results of procedures such as blood work and genetic screening and that many used that data for follow-up conversations with their primary care providers.
But despite its popularity, returning test results is not a routine research practice, said lead author and assistant professor of nursing Denise Kent. Making it standard practice could help bolster enrollment and engagement in clinical studies and trials, particularly from Black and Latino populations commonly underrepresented in those scientific efforts, she said.
“It goes without saying, but in order to conduct human research, we need human volunteers,” said Kent, assistant professor in the colleges of medicine and nursing. “If we ask people to participate in research and we learn something about their health, we need to share it with them. It’s common sense that if we treat people right during the research process, they’ll come back and want to participate in more research.”
The study found that nearly three-quarters of study participants between ages 18 and 65 shared their test results with their primary care provider. Some participants reported starting a new treatment due to those interactions.
video
Most participants said they preferred to get the results through the online portal MyChart. But half of the Spanish-speaking interviewees — one quarter of the study pool — preferred phone. Kent suggested that may be due to language barriers and the opportunity to ask follow-up questions about their results.
That could be related to a finding that nearly one-quarter of the participants reported worry and anxiety after seeing their test results. These stresses show the need for using experienced clinicians, such as emergency/critical care nurses and nurse practitioners, to deliver information and answer questions from study participants, Kent said.
The authors also said this process should be built into future studies to ensure participants receive their results in the most useful and stress-free manner.
“Researchers need to budget and plan before the study starts to return research results effectively and meaningfully,” Kent said. “This shouldn’t be a conversation to have in the middle of a study.”
These engagements with research participants may also help diversify the patient pool for clinical research and add value beyond the usual cash or travel compensation, Kent said.
The additional interactions between participants and study staff also offer further benefits. In her work with the RECOVER study, Kent helped connect participants to affordable health insurance and primary care services at clinics, such as UI Health’s Mile Square Health Center, which provide care regardless of a person’s ability to pay.
“It’s a win-win,” Kent said. “It’s a win for research, it’s a win for science, and it’s a win for UIC in our community.”
In addition to Kent, UIC co-authors on the study include Nathan Tintle and Michelle Villegas-Downs of the College of Nursing; Michael Freedman, Jerry Krishnan and Martha Menchaca from the College of Medicine; and Lynn Gerald and Jenny Sculley from the Office of Population Health Sciences.